Background of Chinese Periodic risk Evaluation Report
NMPA issued guidance “Periodic risk evaluation report (PRER) of medical device” on 02,07,2020. It applies to class II and III of medical device. For class I medical device, only filing of PRER internally is essential.
PRER is also called Chinese periodicic safety updated reports (PSUR) because it is very similar to European PSUR required at MDR. In the fact it is a mixture of a part of European PUSR and risk analysis in term of vigilance (passive) and post market surveillance (active).
NMPA want to strength the monitoring of safety of in China approved medical device and monitor post market surveillance in China and worldwide.
For required PRER in China it contains worldwide marketing history, vigilance summary, literature evaluation and risk analysis related to adverse effects, adverse events and recalls.
PRER for overseas products can be
submitted in Chinese or bilingual:
English and Chinese and will be submitted electronically through Chinese agent
annually.
Timeline of Periodic Risk Evaluation Report
PRER period starts from approval date on NMPA certificate and ends at the same day (ending date), throughout first 5 years annually. The deadline of submitting PRER is within 60 days of ending date of PRER.
How to submit PRERs depending on product classes and how to calculate first 5 years of PRER period, below we give a clear insight.
Frequency of PRER to NMPA
The manufacturers of class I medical device should
- FILE PRER annually (just for intern record) after initial registration (5 times) till next renewal
The manufacturers of class II and III medical device should
- SUBMIT PRER annually after initial registration (5 times) till next renewal
- FILE PRER (just for intern record) since first renewal (once every 5 years)
Example of PRER period
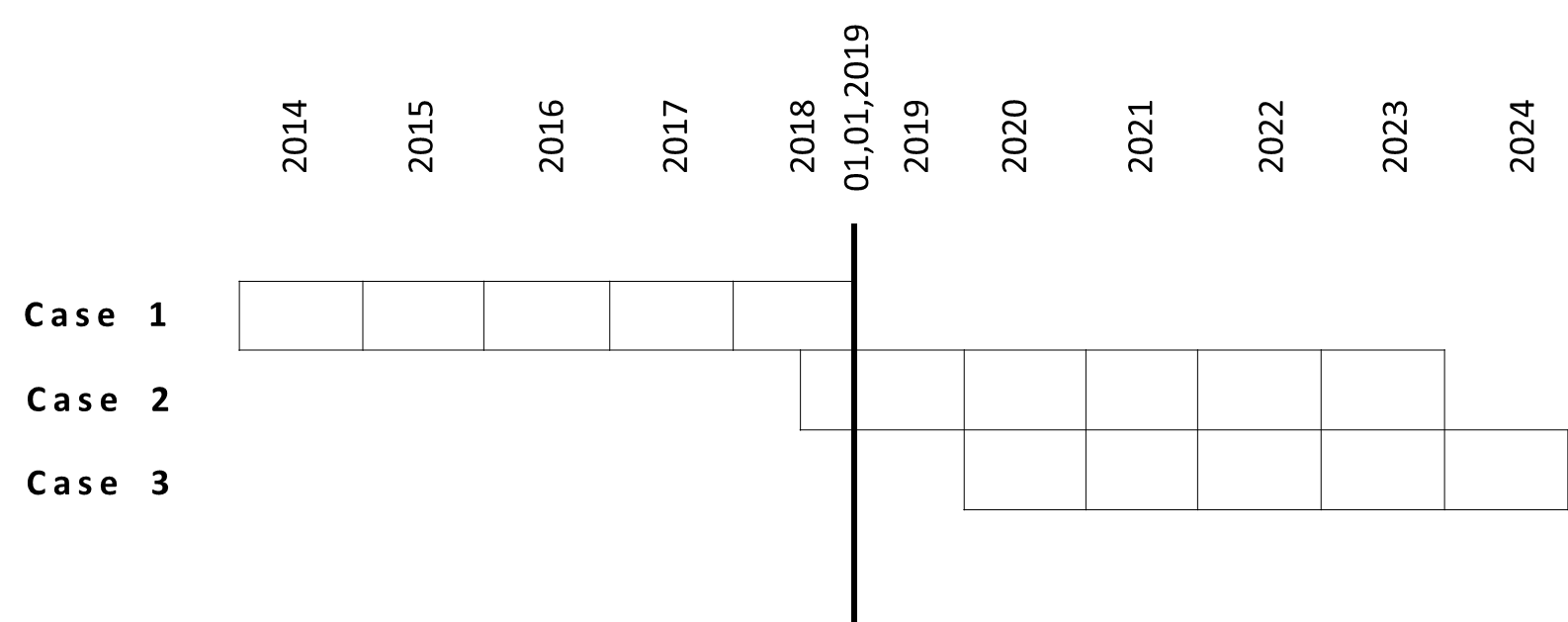
PRER starts always from the fist approval date. Very important date to determine whether PRER applies to is 01, 01, 2019. The reason is that the PRER guidance is valid as of 01, 01, 2019.
Case 1: If first renewal of certificate occurs already before 01, 01, 2019, there is only need to FILE every 5 years one PRER as intern record, not to submit to NMPA.
Case 2: If the NMPA certificate is dated on e.g. 05, 06, 2018, you should submit:
- PRER in 2019: from 05, 06, 2018 to 04, 06, 2019
- PRER in 2020: from 05, 06, 2019 to 04, 06, 2020
- PRER in 2021: from 05, 06, 2020 to 04, 06, 2021
- PRER in 2022: from 05, 06, 2021 to 04, 06, 2022
Ending date of PRER: 05, 06
In the first 4 years the manufacturer has to submit PRER till 04, 09 (within 60 working days of ending date of PRER periodic)
At the 5th PRER it is a bit complicated. Manufacturer has to submit 60 working days earlier before expiration date on the certificate. Normally the manufacturer renews the product to NMPA more than 6 months earlier till expiration date of NMPA certificate. In this case, the 5th PRER ends still 60 days before expiration date.
- PRER in 2023: from 05, 06, 2022 to 04, 03, 2023 (=04, 06, 2023 minus 60 days)
- 2024-2029: only FILE PRER as record etc.
Case 3: If the NMPA certificate is dated on e.g. 05, 06, 2019, you should submit:
- PRER in 2020: from 05, 06, 2019 to 04, 06, 2020
- PRER in 2021: from 05, 06, 2020 to 04, 06, 2021
- PRER in 2022: from 05, 06, 2021 to 04, 06, 2022
- PRER in 2023: from 05, 06, 2022 to 04, 06, 2023
- PRER in 2024: from 05, 06, 2023 to 04, 03, 2024
If manufacturer want to renew certificate early in 04, 03, 2024
- PERE in 2024: from 05, 06, 2023 to 04. 12. 2023 (04, 03, 2024 minus 60 days)
- Date of first renewal --- + 5 years, FILE PRER as record etc.
With the time scale at
figure 1 you can match your product correspondingly.
Analysis of Periodic Risk Evaluation Chapter
Marketing history
It refers to registration information in which model and variants and in which other countries medical device is also approved.
Previous risk control measures
Here is control measures mainly of AE and recall asked.
These measures come from worldwide registered AE and recalls. Besides it, NMPA requires whether
- Withdraw of product certificate (should be a kind of marketing record above)
- Manufacturing process changes
- IFU are revised
- Notifiable change happens
- Clinical re-evaluation due to safety concerns
Summarised if the items above did take place, NMPA should have concerns about the safety of approved products in China. So think about being conservative by reporting. Otherwise you have to provide "countryspecific reasons" why these were not reported or integrated in change registration to NMPA. For manufacturing process changes and revised Chinese IFU you have to make an official application at NMPA in China.
Vigilance (adverse event and recall = FSCA)
Chinese PSUR requires report of individual and group adverse events and recalls worldwide. Actually in many countries the adverse events affecting group is regarding to severity of incidents regarded automatically to high risk event to register to authority.
Risk analysis
Here is focus on cause and control measure of risk derived from registered AE and recall.
The risk analysis in Chinese PSUR sounds for some manufacturers not immediately understandable. Actually periodic risk analysis at risk assessment of incident represents an output of post market activities of approved medical device. Adverse events, recalls or observed incidents of similar product at authority’s vigilance site have to be in risk analysis and adverse effects of medical device in literature should be evaluated. Subsequently, control measurement of these risks should be also analysed.
Template of Periodic Risk Evaluation Report
1. Basic info
- PSUR
- Info of legal manufacturer
- Product name, variant and model
- Product composition
- Intended use
- Certification number
2. Worldwide marketing history
- Country
- Product name, model and variant
- Status of registration (Valid, obsolete)
- Initial approved date
- Desolate date if applicable
3. Previous risk control measures
Yes or no answers in relate to terms below, evidence record if applicable
- Withdraw of approved product
- Recall
- Significant change of manufacturing process
- Revision of IFU
- Design change
- Clinical re-evaluation
4. Worldwide adverse event and recall reporting (individual and group)
- AE reporting number
- Product name, model and variant
- Venue of AE
- Time of AE
- Description of AE
- Control Measures of AE
5. Risk analysis
Summary of safety hazards should be evaluated. If some incidents happen, the cause to this AE (performance or clinical features, design requirement, manufacturing process, storage and transport condition, operating environment) has to be analysed. The control measures are also a part of risk analysis to conclude that these hazards have no safety concerns left.
6. Conclusion
The current PRER compared to last periodic of PRER should be analysed. The acceptance of difference should be evaluated.
Service of Chinese Periodic risk Evaluation Report
In Professional service we guarantee that you pass the final review at authority. We will accompany the delegation of task internallys and communication with your Chinese distributor or Chinese agent.
| Item | Regular | Professional |
|---|---|---|
| Excel template or word template | x | both |
| Instruction of template | no | x |
| Translation to Chinese, back up in English | no | x |
| Kick off meeting | no | x |
| Project management (intern and extern) | no | x |
| Warranty of passing the review at authority | no | x |
| Price in EUR | 99 | 149 |
Regular service
Professional service
Comparison to Periodic safety update report PSUR at MDR (Article 86)
Compared to EU PSUR there are some limitations of Chinese PSUR in terms of state of the art:
- No expiration of Chinese PSUR (so far no explanation about cancellation of certificate or no disstribution of medical device)
- No data of similar product
- No data from official database (as EU register)
| Items | Periodic risk evaluation report in China | Periodic safety update report in Europe |
|---|---|---|
| Product class | I, II, III | IIa, IIb and III |
| Frequency of report | Annually after initial product approval (5 years) | Class IIa: every 2 years; Class IIb and III: every year |
| Report to | NMPA in electronic format | Part of technical documentation. Class IIa, IIb and III: Report to Notified body, on demand to authority competency Class III: additionally at EUDAMED technical documentation. |
| Key contents at report | Worldwide marketing history, worldwide incident reporting and literature evaluation of vigilance, risk analysis (trend analysis, adverse event, recall, adverse effect, CAPA) | Worldwide marketing history, incident reporting, trend analysis, customer complaints, monitoring of similar products, literature evaluation, CAPA, PMCF, risk analysis and benefit to risk analysis in term of incident |
Some thoughts about Periodic Risk Evaluation Report
NMPA has elite tempo to strength registration complexity of medical device since 2014. NMPA is aware that at submission dossier there is no post market information. After issuing guidance of adverse event and recall in 2019, Periodic risk evaluation report is born in 2020.
Actually European PSUR of medical device copies from PSUR of drugs focusing adverse effects. It is kind of mishmash of PMS required for medical device, one of the upregulation at MDR in Europa. China follows PSUR quickly adding requirements of risk analysis with new name Periodic risk evaluation report.
As shown in template there is too wide extent at risk analysis which related to registered adverse events, recall and co. We highly recommend you not seriously to report sensitive CAPAs, revision of IFU outside China, design change etc. Otherwise it raises concerns by NMPA that medical device has safety problem worldwide. Instead, provide complete worldwide marketing history, adverse events, recalls and its cause and control measurement in term of risk analysis.
Unfortunately NMPA is drafting already new guidance regarding post market surveillance, and PMCF follows:)
Trend of international PSUR
· Consolidated template of international PSUR
· Process of international PSUR
· Maintenance of KPI data needed for diverse PSUR
· Digital tool for automatization of international PSUR
Taiwan
It is called 醫療器材安全性總結報告(Safety Summary Report, SSR). It is very rational that only high risk class III device as stent, Heart valves, artificial joints which are initially registered in Taiwan is applicable for a regular safety report
International
The Medical Devices (Post-market Surveillance Requirements) [1]
(Amendment) (Great Britain), 2025
EU 2017/745, MDCG 2022-21 - Guidance on Periodic Safety Update Report[2], 2022
EU EMA, Periodic safety update reports (drug)[3]
[1] https://assets.publishing.service.gov.uk/media/67813b39363ac763c8ede498/Medical_devices_periodic_safety_update_report__PSUR__formatting__1_.pdf
[2] https://health.ec.europa.eu/latest-updates/mdcg-2022-21-guidance-periodic-safety-update-report-psur-according-regulation-eu-2017745-december-2022-12-16_en
[3] https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/pharmacovigilance-post-authorisation/periodic-safety-update-reports-psurs
Outsourced regulatory affairs manager
If your marketing holder either in China or in other markets is disributor without regulatory knowledge, it makes sense to outsurce RA to us.
Annual (self-examination) quality management system report
Another similar report is Annual (self-examination) quality management system report per chinese agent. There are more KPI to collect for all in China approved medical device.
Post market surveilance in China
Do Not ignore other activties after product approval in China.
International quality management system
What are the international requirements in terms of QMS? It is different with frequency of report, focused content in report and relation to product certificate.
Do you want to be familiar with other post market activities, be expert of chinese and global registration?
Book our E-Learning. We have still discount!