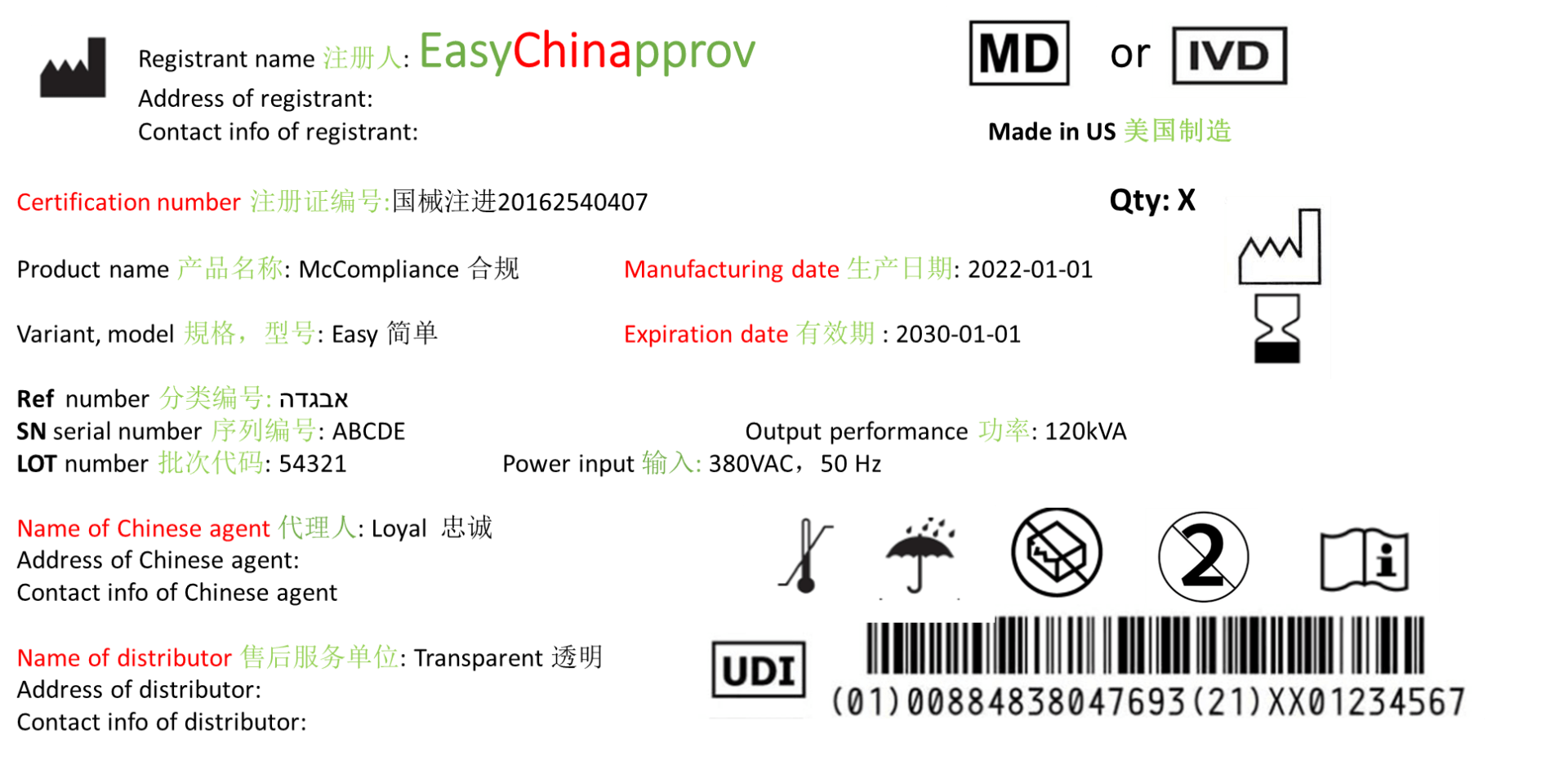
Chinese label of medical device and IVD

Background of Chinese label
The Chinese label of medical device an IVD on product and package is critical in the whole product cycle and can be inspected and examined by Chinese authority NMPA, goods acceptation at hospital and at customer duty after the product label at registration is submitted and approved by NMPA.
The example of Chinese label below is in accord to “Instruction for Use and Labeling of Medical devices” (No. 6, 2014).
Requirements from article 13 Guidance on instruction for use and label of medical device, No. 6, 2014
1. Product name, model and specification
2. The name, address and contact information of the registrant or filing person, and the name, address and contact information of the agent for imported medical devices shall also be indicated
Note: registrant or filing person are legal manufacturer of class II/III or I medical device an IVD. Agent is Chinese authorised representative.
3. The number of the medical device registration certificate or the record certificate number
4. The name, address, production address, contact information and production license number or production record certificate number of the production enterprise, and the name, address, production address, production license number or production record certificate of the entrusted enterprise shall also be marked with the entrusted production serial number
Note: If medical device was manufactured in China, production number is needed.
5. Production date, service period or expiry date
Note: There is only Chinese symbol for production date and expiry date. So validity is possible as alternative for expiration date.
6. Power connection conditions and input power
7. Graphics, symbols and other relevant content that should be marked according to product characteristics
Note: Symbol should be in compliant to YY/T 0446: 2014 which contents only parts of symbols in ISO 15223-1: 2022. Additional symbols in home country as CE and can be unchanged if there was no Chinese requirements. However if the symbols raises a potential risk, it should be translated in Chinese on label otherwise it could be translated at IFU.
8. Necessary warnings and precautions
Note: Due to potential risk it is recommended to translate warnings and precautions in Chinese language on device or on components.
9. Special storage, operating conditions or instructions
10. For medical devices that have damage or negative impact on the environment during use, their labels shall contain warning signs or warning instructions in Chinese
Note: in Chinese language on device or on components
11. For medical devices with radiation, the labels shall contain warning signs or warning instructions in Chinese.
If the medical device label cannot fully indicate the above-mentioned content due to the limited location or size, at least the product name, model, specification, production date and use period or expiration date should be marked, and the label should clearly state "For other contents, see the instruction manual".
Beyond labelling guidance and symbol standard
· Unique Device Identification (UDI) is new, so far (March, 2022) it is obligatory for all class III medical devices in all levels of packages in China and on device itself.
· Wording: it is tricky that even different wording with same meaning of defined Chinese terms could raise incompliance. So please follow exact existing Chinese term in Chinese regulation of labelling and co.
· Size and layout: there is no fixed size and layout of Chinese character and Chinese symbol
· The obligatory info at example label can be divided in many smaller labels if it was convenient to have many labels on device than all on one label.
· Format of date: normally you can chose x年y月z日 or yy-mm-dd, oft it is precise enough to note date to month.
· Certification number and manufacturing date (see example above) marked in red at example of Chinese label must be added only after product approval. The certification number should be consistent at label and on Chinese certificate of medical device and IVD. The manufacturing date should not in prior to issued date of Chinese certificate
· Please reference product specific guidance and respective standard where Chinese label and symbol are additionally product-related required.
How can we support
Contact us to check your first label for free. Including service of “easy Chinese label” we prove also the process of Chinese label if needed and provide check sheet with Chinese term with English translation, table of label requirements and list of Chinese symbol in YY/T 0446: 2014 for your own label management of medical device and IVD.





